Population Health
Sysmex’s Clinical Data Repository (CDR) platform, Eclair, enables secure access and sharing of diagnostic data across regional and national systems, for clinical care and population health management.
COVID-19 National Solutions
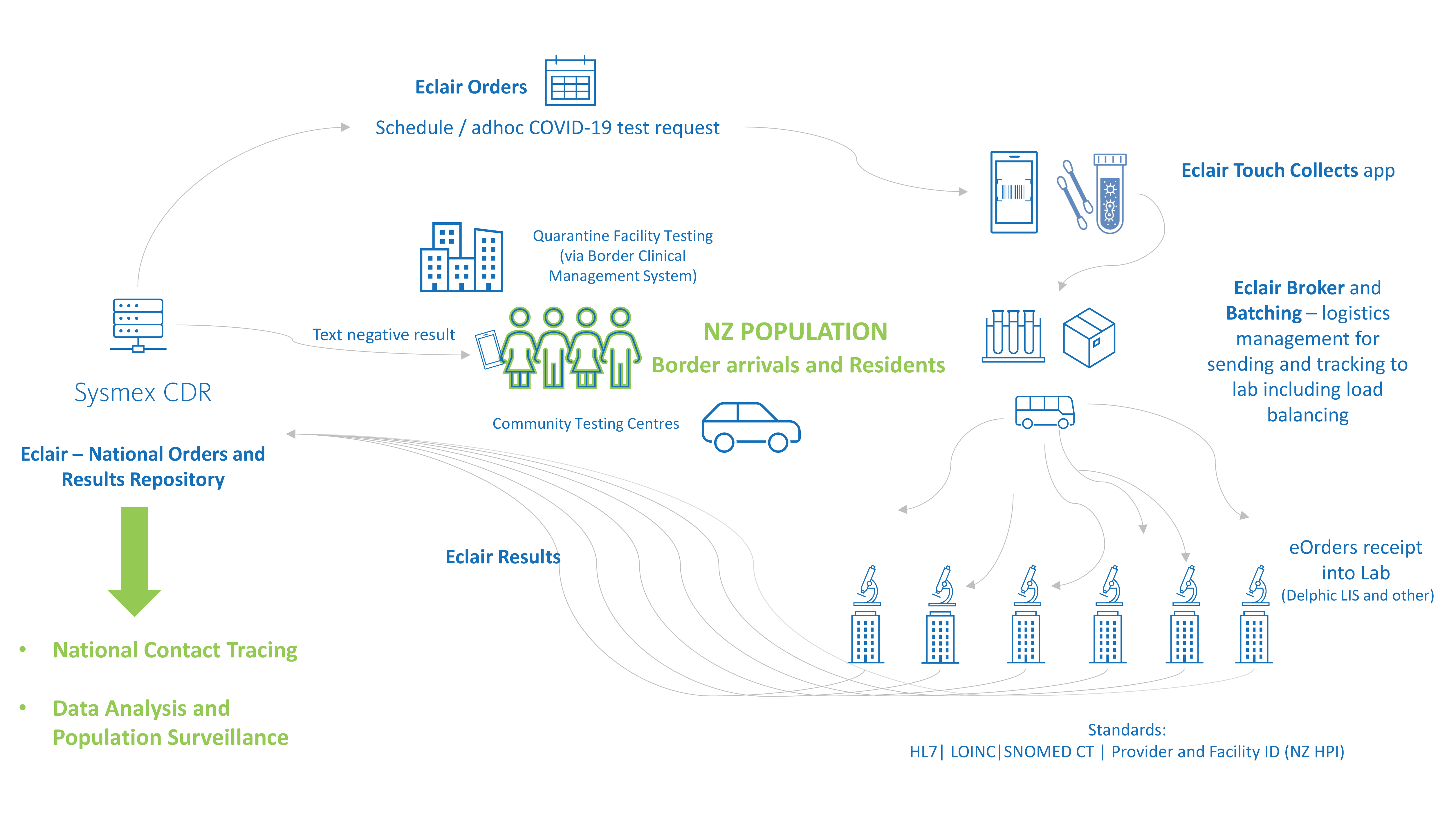
The New Zealand response to COVID-19 is a great example of the power of Eclair, aggregating population testing data and digitising the border management and community testing workflow.
At the heart of the population health management solution is the Sysmex CDR, Eclair which was implemented national notifiable disease centre (ESR – Environmental and Scientific Research) as a key initiative for New Zealand government response to the COVID-19 pandemic.
New Zealand is also implementing a fully digital, national border system, making it a world leader in this space. The paperless border system records and links people’s arrivals into the country throughout their managed isolation stay, including the scheduled COVID-19 testing requests and results for national reporting.
Sysmex has developed solutions to support the paperless testing workflow for all laboratories for the national border system as well as community testing centres for all New Zealanders.
Test Requests
Paper-based COVID-19 test orders present many challenges for data accuracy and can delay processing. To minimise these issues, COVID-19 test requests are centralised electronically in the national Eclair repository. eOrders can be created in Eclair for community testing or via an api from the Border Clinical Management System (BCMS) for guests in quarantine facilities. This enables traceability and accuracy for all tests throughout the pre-analytical workflow.
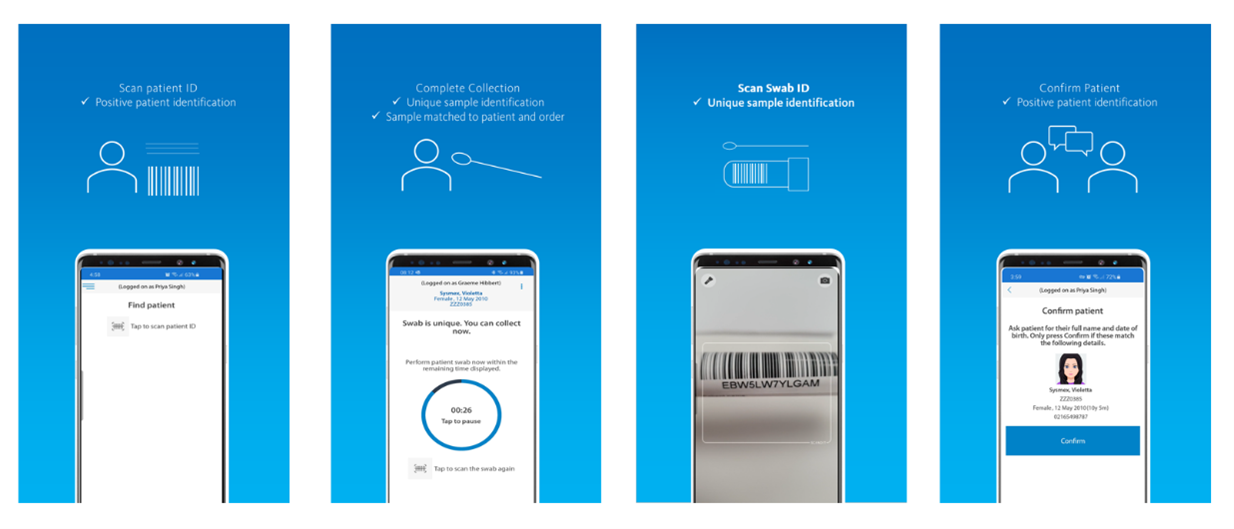
Swab Collection
The purpose of this app, Eclair Touch Covid-19, is to digitize the collection of COVID-19 test swabs – accurately linking the test order, the patient and the specimen through barcode scanning and confirmation of the identification of the patient. The information is securely stored in the national orders and result repository.
Managing Logistics for Testing Labs
To support surges in testing volumes and to enable balanced workload between diagnostic labs, Eclair provides a broker system which can intelligently determine which laboratory will process batches of COVID tests, either from a quarantine facility or community testing centres.
Tracking the transfer of specimens from point of collect to the assigned laboratory is an important part of the solution. Upon arrival at the testing lab, manual data entry is not required as the batch of swabs can be received into the LIS as an HL7 order message from Eclair. This means swabs can be processed faster and accurately. This solution saves significant time and effort for laboratory testing teams during outbreaks when testing volumes surge.
Testing Data and Results
All laboratories across New Zealand that are performing SARS-CoV-2 PCR testing interface to the repository (via HL7) to enable a complete representation of the data set for health intelligence initiatives. The data integrates with New Zealand’s contact tracing system and guides daily reporting by the Ministry of Health executives. Testing data is also used for surveillance analysis, for example linking population demographics to testing patterns such as location and volume.
References
- https://www.stuff.co.nz/national/health/coronavirus/124118119/covid19-the-emotional-weight-of-crunching-coronavirus-case-numbers
- https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(20)30225-5/fulltext
- NZ implements digital border system – Health Informatics New Zealand (hinz.org.nz)
- COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study | ESR
- Streamlining COVID-19 laboratory data to Ministry of Health | ESR
- https://www.health.govt.nz/system/files/documents/pages/mid-term_national_testing_strategy_covid-19_june_2020.docx
- https://www.hinz.org.nz/page/Webinar-19August2020