Sysmex to Begin Offering Laboratory Testing Service for Research Involving the Risk of Recurrence of Early-Stage Breast Cancer
Sysmex Corporation (HQ: Kobe, Japan; President: Hisashi Ietsugu) will begin offering in Japan in January 31 a new laboratory service (for research) related to the risk of recurrence of early-stage breast cancer.
In recent years, changing eating habits and other lifestyle changes in Japan have led to ongoing increases in the number of breast cancer patients. Determining the post-operative risk of recurrence is considered to be an important indicator in the treatment of breast cancer. The selection of treatment methods involves an overall judgment based on a variety of test results. Anti-cancer drugs and other treatments are employed after determining the post-operative risk of recurrence.
Sysmex’s new laboratory service for research (C2P Breast) will involve a new technology (C2P: Cell Cycle Profiling) developed through joint research and performance evaluation tests conducted with university hospitals and other institutions. Measurement will involve studying the expression amounts of two types of proteins included in tumor tissue removed from the patient as well as their enzyme activity. In principle, these results will then be categorized into three stages (H: High; I: Intermediate; and L: Low).
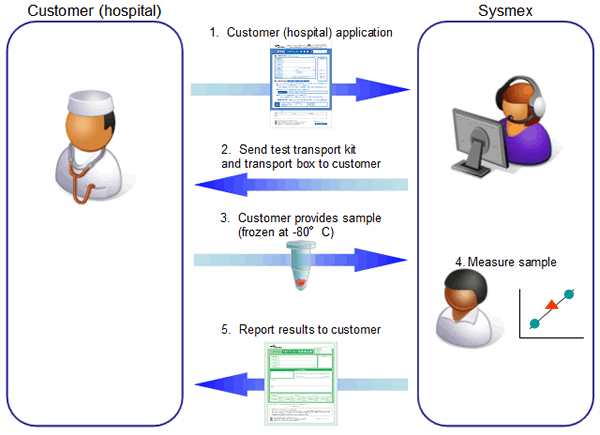
Our procedure for undertaking this service will be to have customers (hospitals) send us frozen samples of patients’ tumor tissue in special test transport kits. Sysmex will measure the samples and report the results to customers (hospitals). We expect to report these results within two to three weeks of receiving the samples.
Sysmex’s Group Mid-Term Management Plan includes three core strategies*. Based on one of these core strategies, “Innovating Life Science,” we aim to create proprietary testing technologies in the life sciences field, expanding our operations in cancer and other areas of molecular diagnostics.
To date, Sysmex has developed a highly precise technology (the OSNA method, for One-step Nucleic Acid Amplification) for determining the existence of breast cancer lymph node metastasis in only around 30 minutes. We have introduced products employing this technology at numerous healthcare facilities.
Going forward, Sysmex will continue working to develop and promote high-value testing that will contribute to improvements in quality of life (QOL) for patients, the standardization of medical services and the development of personalized medicine.
[Overview of Laboratory Testing Service]
(1) Name: C2P Breast
(2) Target market: Japan
(3) Price: ¥200,000/test
(4) Flow of contracted testing:
Terminology – Three core strategies:
(1) Leading Hematology
Sysmex has become a global leader in the field of hematology by providing products with high levels of quality and usability, as well as advanced after-sales support, in more than 170 countries throughout the world. Going forward, we plan to further leverage our collective strengths to seize the position of undisputed global leader.
As the industry frontrunner, by applying advanced technologies and through ongoing R&D initiatives, we aim to create new value in the area of blood disorder diagnosis. At the same time, we will continue to grow through our leadership role in next-generation hematology.
(2) Leading in Emerging Markets
As the sole global player in the IVD field that is based in Asia, Sysmex aims to be the leading company in Asia in this field.
Leveraging the advantages we have cultivated in Asia, we aim to become a comprehensive IVD supplier in emerging markets-which are expected to grow rapidly-by launching products tailored to market needs and enhancing our sales and support networks.
By reinforcing our advantages in Asia, we aim to take a leading position in this region’s IVD market. We will also lead the development of diagnostics markets within these countries.
(3) Innovating Life Science
In the Life Science, we aim to create unique testing techniques employing new technologies in the area of Theranostics, particularly for cancer.
By combining our own technologies with those from others, we engage in initiatives to create new value in the Life Science, such as integrating personalized medicine with treatment and diagnosis and we aim to secure a global position.

